Abstract
Background: A retrospective cytogenetic study encompassing 5290 patients with chronic lymphocytic leukemia (CLL) identified complex karyotype (CK) as adverse prognostic factor. In cases with ≥5 chromosomal aberrations (CAs), this finding was independent of TP53 alterations (Baliakas et al., 2019) Which chromosomal rearrangements dominate CK-CLL is insufficiently defined impeding the identification of genomic events responsible for poor prognosis. To delineate cytogenetic aberrations in CK-CLL, we analysed conventional karyotypes from 125 patients classified as high-risk during the era of chemoimmunotherapy.
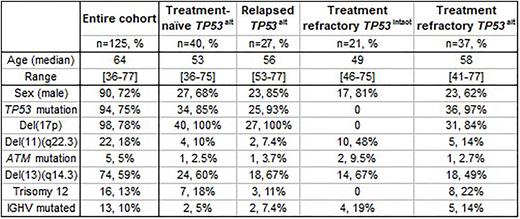
Methods: Chromosome banding (CBA, CpG oligonucleotide and IL-2 stimulation, N=131), SNP-array analysis (Affymetrix 6.0, N=109), DNA methylation analysis (Illumina Infinium HumanMethylation450 BeadChips, N=45) and telomere length measurement (N=122) was conducted on samples taken at enrolment on the CLL2O trial of the GCLLSG/FCLLSG (NCT01392079). This trial encompassed CLL patients (pts) with TP53 alteration (deletion/mutation, TP53alt; treatment-naïve or purine-analogue relapsed/refractory) and TP53intact purine-analogue refractory disease. CBA and TP53 mutation data from the CLL11 trial of the GCLLSG (NCT01010061, N=152) was integrated for multiple linear regression.
Results: An informative karyotype was obtained in 125/131 pts (for clinical/genetic features see Table). The total number of CBA-detected CAs was 618 with an average of 4.9 CAs per case (range 0-21). Pts with TP53alt had a significantly higher karyotype complexity than pts without (5.3 vs 2.7 CAs mean, p=0.02). A normal karyotype was found in 2 pts, a non-CK in 32 pts (≤2 CAs; 20 pts TP53alt), a low- or intermediate-CK in 34 pts (3 or 4 CAs, 30 pts TP53alt), and a high-CK in57 pts (≥5CAs, 54 pts TP53alt).
Cytogenetic aberrations comprised 341 derivative chromosomes, 131 interstitial/terminal losses, 65 monosomies, 53 balanced translocations, 21 trisomies, 5 inversions, and 2 interstitial gains. Remarkably, 308/618 CAs had at least one breakpoint in the centromere (p10/q10; N=70) or in p11/q11 (N=238) directing our interest towards (peri-)centromeric breakage. Chr17 was most frequently affected linking (peri-)centromeric breakage to TP53 loss. Whole arm translocations (WAT) constituted the most common cause for TP53 loss (N=47 in 39 pts) with chr18 (N=9), chr17 (N=6), chr8 (N=6) and chr15 (N=6) being the most frequent translocation partners. Other cytogenetic events underlying TP53 loss were complex unbalanced rearrangements (N=40 in 39 pts), simple interstitial/terminal deletion (N=22 in 22 pts) and dicentric chromosomes (DIC, N=8 in 8 pts, 6 with breakpoints in p11/q11). In total, 98/117 aberrations causing TP53 loss had breakpoints in (peri-)centromeric cytobands. Mapping chr17 breakpoints based on SNP-array results revealed that more than half (58%) clustered within a 1 Mb region on both sides of the centromere. In contrast, chr17 breakpoints in CK-AML are random suggesting that chr17 (peri-)centromeric instability is specific for high-risk CLL.
(Peri-)centromeric rearrangements in form of WAT and DIC were also found to occur without chr17 involvement (N=24 in 20 pts and N=12 in 8 pts, respectively). Chromosome involvement was random pointing towards general (peri-)centromeric instability. Multiple linear regression revealed only karyotype complexity but not TP53alt or prior treatment as significant risk factor for WAT/DIC substantiating the notion that (peri-)centromeric instability is an intrinsic hallmark of high-risk CLL.
DNA methylation patterns in regions adjacent to pericentromeric breakpoint clusters located on chr17p and chr18p showed no abnormalities when compared to cases without chr17/chr18 aberrations. Telomere length was significantly shorter in cases harboring at least one (peri-)centromeric rearrangement (p<0.001), but this association could not be confirmed when restricting the analysis to non-CK cases (N=20 vs 13, p=0.08).
Conclusions: Karyotype complexity was confirmed as typical feature of high-risk CLL. (Peri-)centromeric aberrations showed up as new hallmark characteristic of CK-CLL and WAT/DIC as otherwise rare events in hematologic malignancies were frequently observed. The underlying reasons for (peri-)centromeric breakage and its prognostic impact beyond karyotype complexity and TP53 alterations require further investigation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.